Upcoming regulatory changes to Natural Health Products (NHPs)
Stephanie recently surveyed all Calgary Midnapore households about whether the regulation changes to NHPs the Liberal government included in Bill C-47 would affect them, and how. There was significant feedback and, while responses are still trickling in, the results to date have been summarized (charts below).
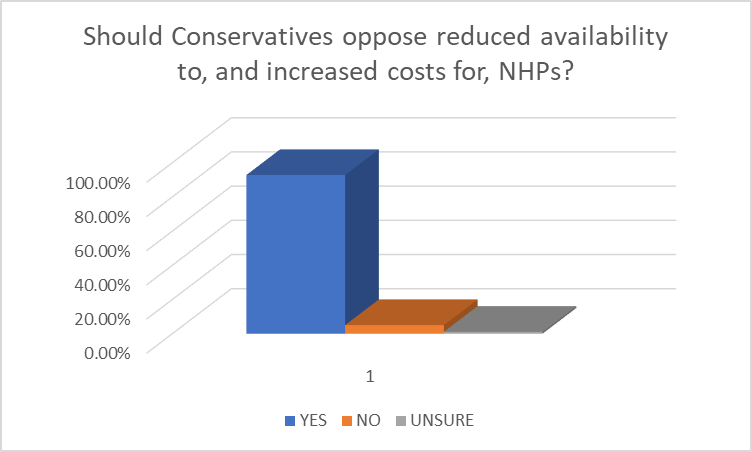 (Charted results of the 93% of respondents (identified constituents) who either use NHPs themselves or have a family member who does).
(Charted results of the 93% of respondents (identified constituents) who either use NHPs themselves or have a family member who does).
To clarify, Bill C-47 changed the definition of therapeutic product to include NHPs in some instances, but not in other key situations (such as allowing additional assessment, testing, studies and adverse reaction reporting). Below is the Library of Parliament’s legislative report on the relevant section of C-47.
Clauses 501(1) and 502(1) introduce a definition of “therapeutic products” that apply to particular sections of the FDA, notwithstanding the definition in section 2. In both cases, “therapeutic products” are defined to “not include a natural health product.” Specifically, clause 501(1) introduces section 21.321, excluding “natural health products” from the Minister of Health’s power to require assessment of the therapeutic product (section 21.31) and the minister’s power to order additional tests or studies on therapeutic products (section 21.31). Clause 502(1) introduces section 21.8(2), which excludes “natural health products” from a health care institution’s requirement to report serious adverse drug reactions that involve a therapeutic product.
Since that mailing, Stephanie has met with representatives from the Canadian Health Food Association (CHFA) who shared their concerns about how they view this as over-regulation, and how it will potentially put Canadians at risk.
As they explained, the government is reverting back to antiquated labelling standards, which have both an environmental impact, and a cost impact to companies. Additionally, the fee program is being updated with little consideration for the effect on the industry. For example, instead of a gradual implementation, the price to related to developing new products will jump by 7000 per cent in 2025 (from $7000 to $56,000), discouraging companies from expansion and development at best, and making it impossible at worst. Costs associated with labelling changes and these new development fees will result in some suppliers just folding entirely, and others raising costs to consumers.
Canada currently is the only country which regulates NHPs and is considered internationally to be an example of an extremely safe industry. However, there is a loophole which allows Canadians to order NHPs and supplements to be imported from non-regulated countries. If domestic prices increase and selection decreases, the CHFA has legitimate concerns that Canadians will resort to purchasing unregulated products which could have significant adverse health impacts.
There has been an industry report on the issue released recently, and it confirms the level of alarm about how widespread the impacts will be. Conservatives will continue to push the Liberal government to reconsider the impacts to consumers of this increased regulation.